Explain Why Isotopes Have Different Mass Numbers
This can happen because although the number of protons in. Since the isotopes have the same number of protons and electrons the isotopes have much the same chemical behavior.
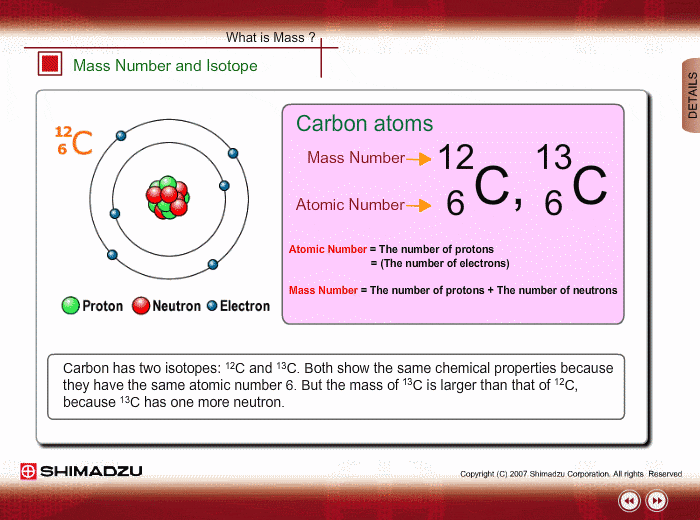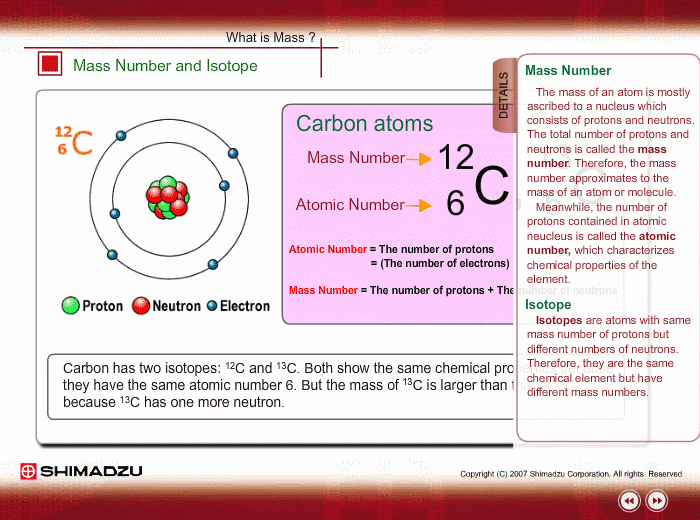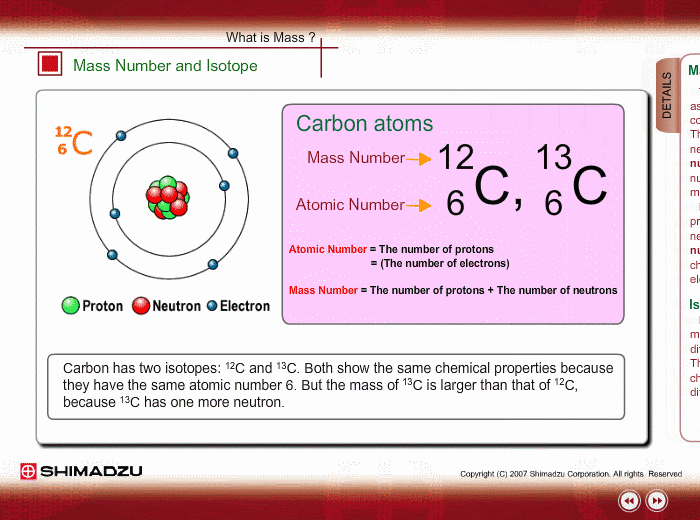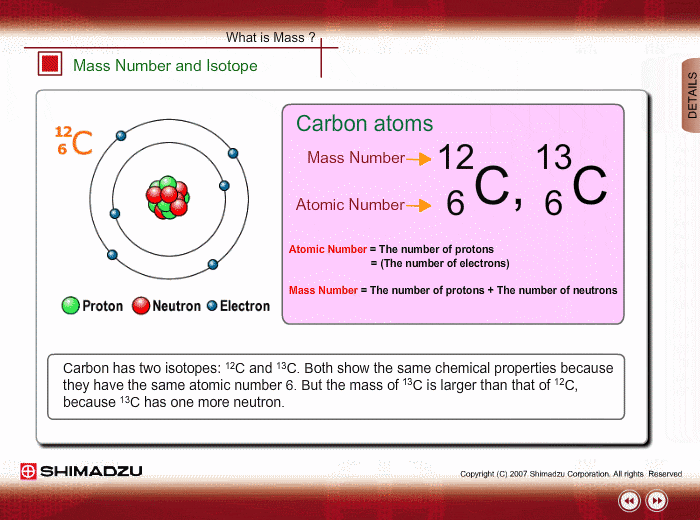
Mass Number And Isotope Shimadzu Shimadzu Corporation
Those elements that have the same atomic number but a different mass number are referred to as isotopes.
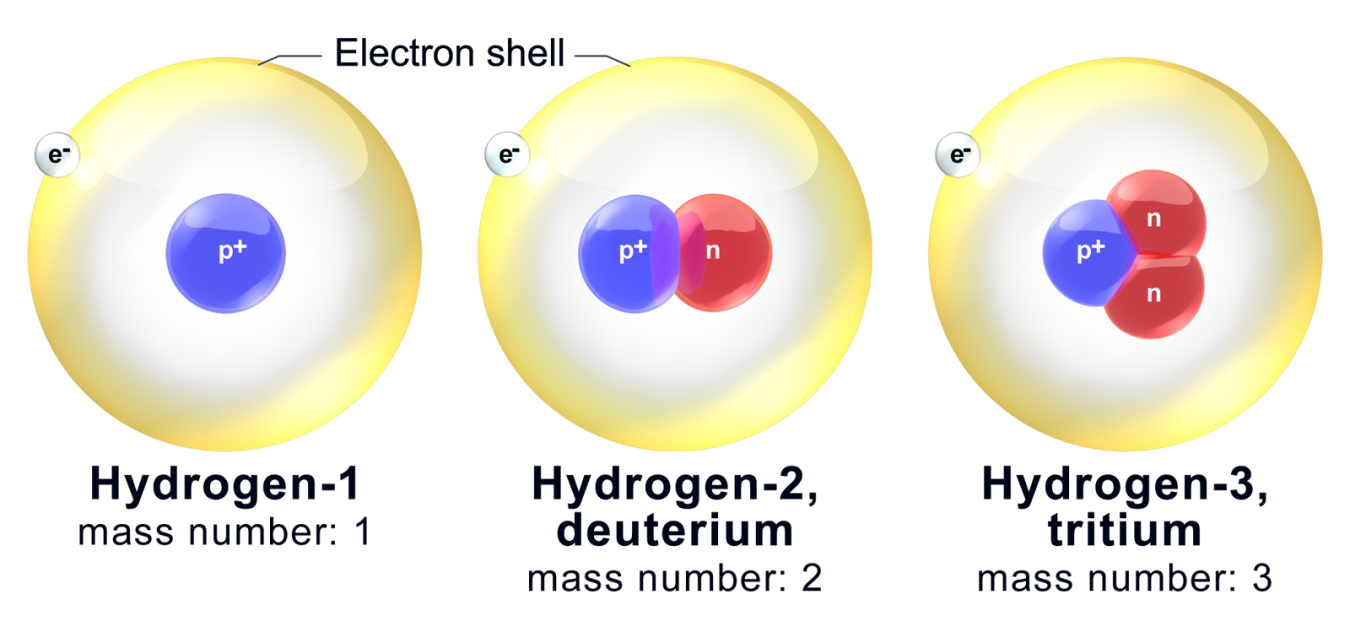
. The atoms of a chemical element can exist in different types. They have similar chemical properties because isotopes of an element have the same number of electrons as an atom of that element. Mass number determines the physical properties while atomic number determines the chemical.
So it is clear that they will have same number of electrons same electronic configuration and thereby same number of valence electrons. Explain why these atoms have different mass numbers. Atoms of the same element having the same atomic number but different mass numbers are called isotopesIsotopes have same atomic number but different mass number because they contain.
All atoms of a given element have the same number of protons hence the same atomic numberThey may however differ from one another in mass and therefore in mass number. Why do isotopes have same atomic number but different mass number. Isotopes are atoms with the same number of protons but that have a different number of neutrons.
Start your trial now. Experts are tested by Chegg as specialists in their subject area. Different isotopes of the same element have different masses.
Since in chemical reactions it is the valence electrons which participate so we can conclude that isotopes have similar chemical properties. For example Hydrogen H exists in the form of three isotopes. Of electron or proton is same in all atoms that.
35 - 17 18 neutrons. Write the two postulates of. The different atoms of the same element having same atomic number but different mass numbers.
Submitted by chemistry123 on Mon 09192011 - 1620. The electron arrangement is the same owing to same chemical properties. Since the atomic number is equal to the number of protons and the atomic mass is the sum of protons and neutrons we can also say that isotopes are elements with the same atomic number but different mass numbers.
Such atoms of the same elements are called as isotopes of the element. First week only 499. However they have different numbers of neutrons which affects the mass number.
For example hydrogen is an element in the periodic table with atomic number 1 and mass number 1. Although carbon-12 weighs exactly 12 amu the periodic table reports that the mass is 12011 because we are taking into. Weve got the study and writing resources you need for your assignments.
Please note that the difference in the mass numbers of the isotopes is because of the difference in the number of neutrons present. The reason for this is because isotopes of an element have the same number of electrons as an atom of that element but they have different number of neutrons which affects the mass number. Isotopes have the same atomic number but different mass.
Ŷuŵďer saŵe of eleĐtroŶsͿ Thats ǁhy they haǀe saŵe chemical properties while the atom of the same element has different number Mass number different of neutrons therefore they have different physical. Iso means the same like in triangles isosceles so the atoms are the same element but different in mass. We review their content and use your feedback to keep the quality high.
Isotopes are atoms of an element with the normal number of protons and electrons but different numbers of neutrons. Isotopes have the same atomic number but a different mass number. Since hydrogen atomic number is 1 and its isotopes dutarium and tritium have 2 and 3 mass number.
The increase in mass due to neutron which doesnt effect the chemical reaction since electron and proton are same so its only physical properties become different that is massdensity etc. The masses on the periodic table are the average mass of all isotopes and their abundances found in the universe. Explain with the help of an example.
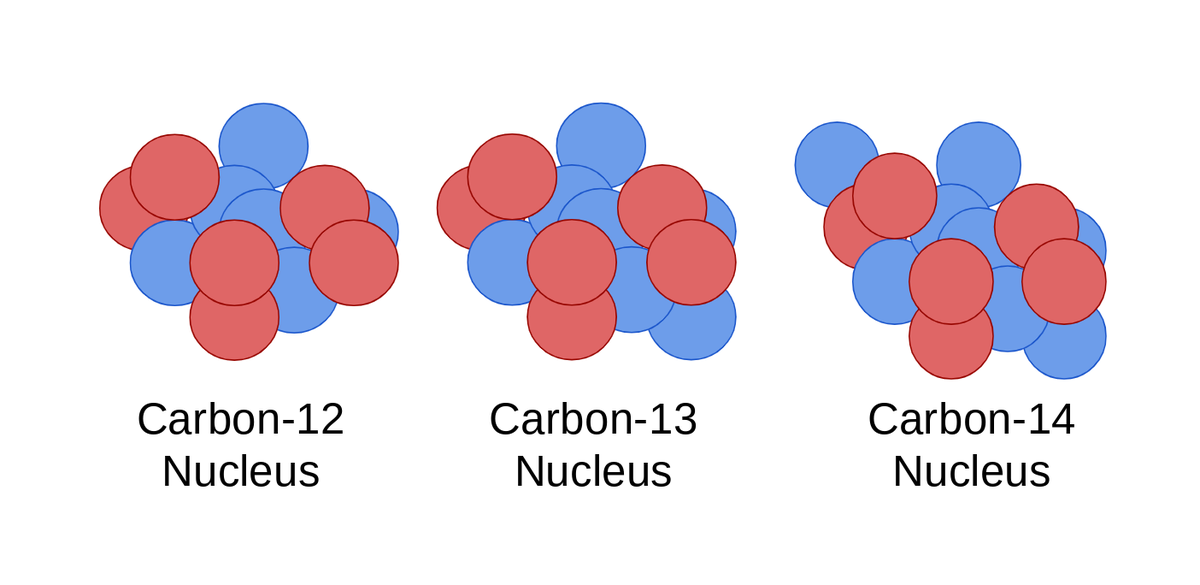
Solution for What are the different mass numbers of Isotopes. One of the best known isotopes is C14 6. The nuclei of Three naturally occurring magnesium isotopes have the same number of protons but different numbers of neutrons.
The mass number protons and neutrons varies because isotopes of an element have. This is because the number of electrons determines chemical properties and all three isotopes have one electron in their atoms. Dutarium is used in nuclear reactor too.
Mass number refers to the sum of the protons and neutrons in the nuclei of the atoms of the element. These are named as. Isotopes occur due to the presence of a different number of neutrons in elements having the same atomic number as mass number is the sum of.
There are different ways of stabilizing the protons there are different isotopes. All the Isotopes of an element have identical chemical properties. An element may have the same number of neutrons or may have more number of neutrons than the number of protons and electrons.
Step-by-Step 1 Isotopes are the atoms with same. Who are the experts. Different isotopes have different mass numbers because they have different numbers of neutrons.
Isotopes are the atoms of an element which have same atomic number but different mass number. They have the same number of protons and electrons but different numbers of neutrons. Isotopes are two or more types of atoms that have the same atomic number number of protons in their nuclei and position in the periodic table and hence belong to the same chemical element and that differ in nucleon numbers mass numbers due to different numbers of neutrons in their nuclei.
Since the isotopes have different numbers of neutrons the nuclear behavior differs. The number of neutrons varies for an element. These are called isotopes.
1 Carbon has th ree isotopes 6 C 12 6 C 13 and 6 C14.
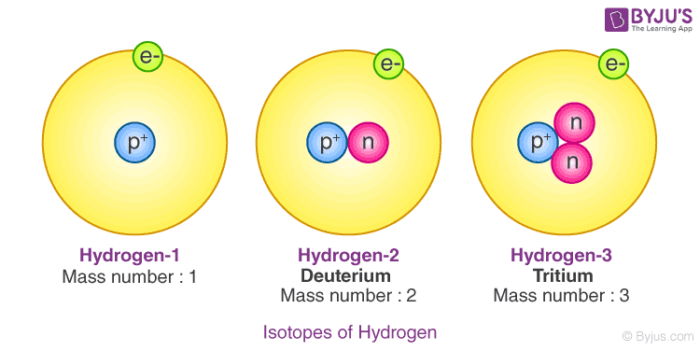
Isotopes Of Hydrogen Plutonium Deuterium Tritium With Examples Videos
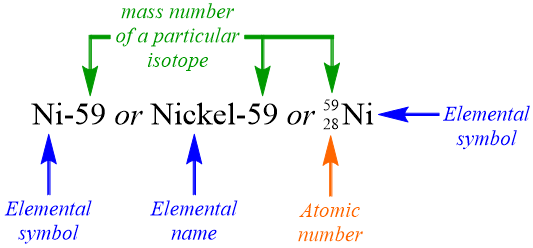
2 4 Neutrons Isotopes And Mass Number Calculations Chemistry Libretexts
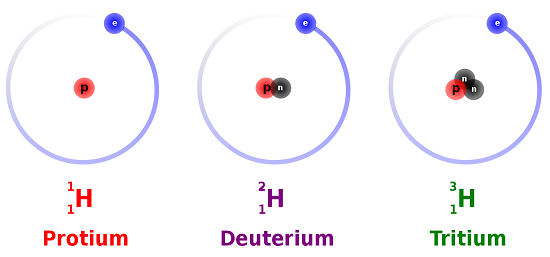
5 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts

What Is An Atomic Number Definition And Examples
Atoms Isotopes Ions And Molecules Boundless Biology
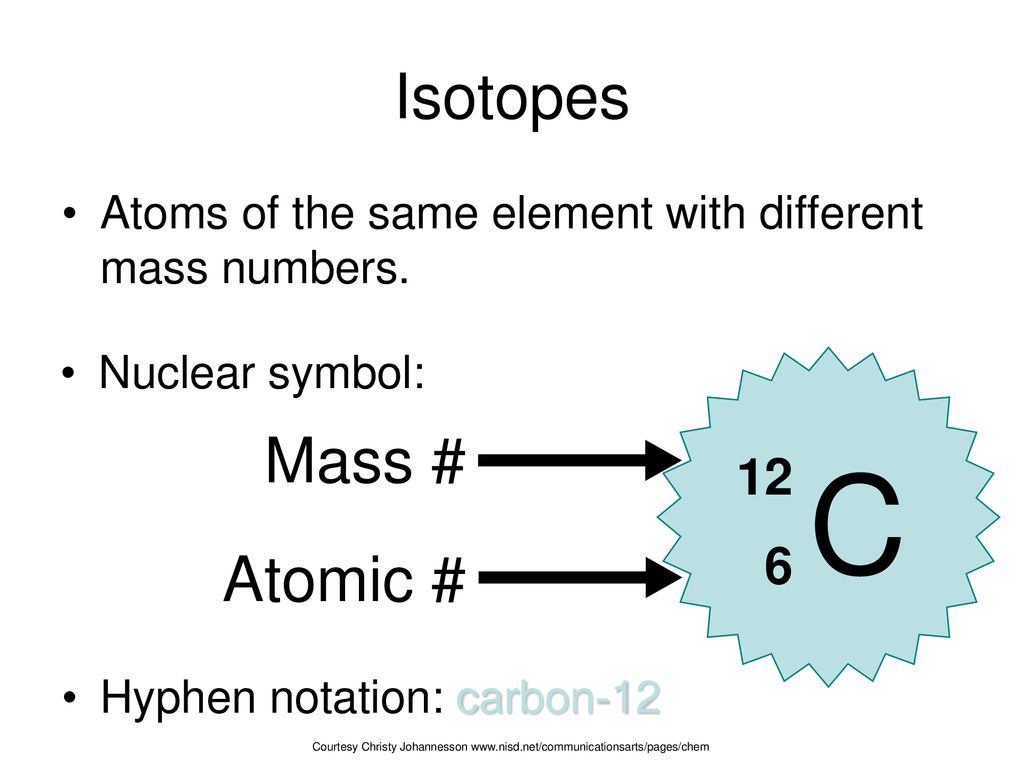
C Isotopes Mass Atomic Ppt Download

Lesson Explainer Isotopes Nagwa
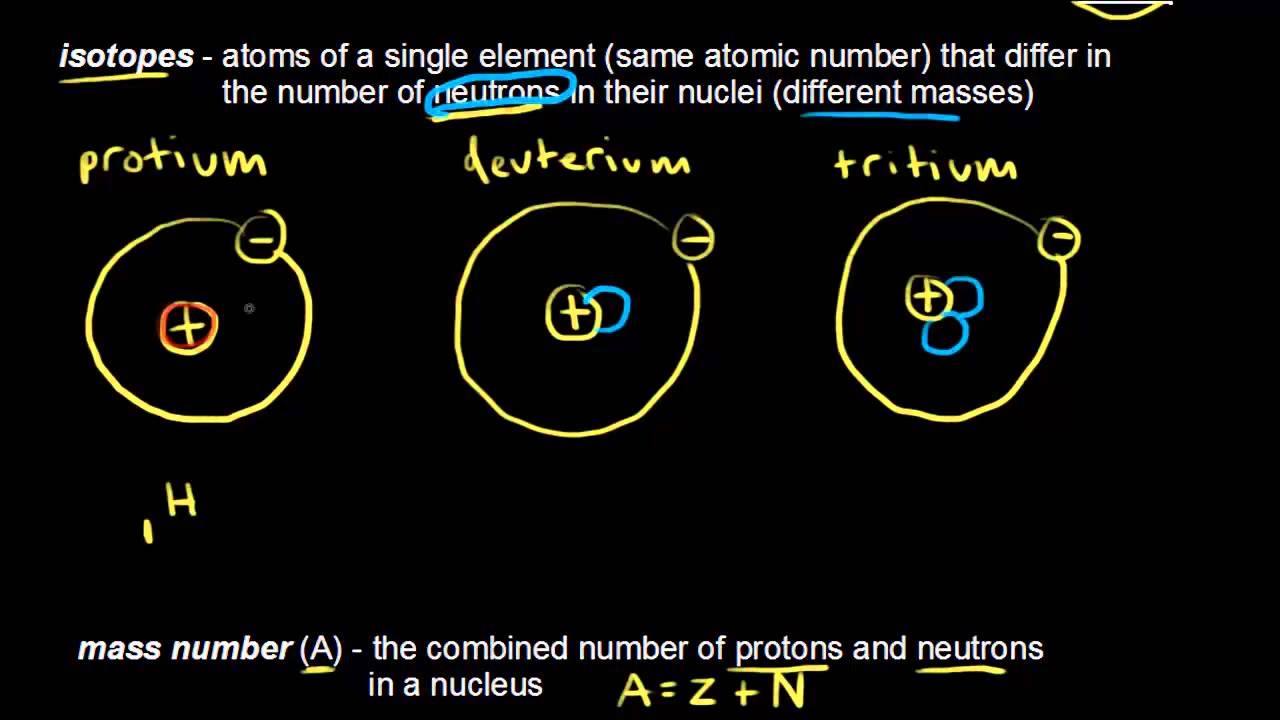
Atomic Number Mass Number And Isotopes Video Khan Academy

Doe Explains Isotopes Department Of Energy

Mass Number Definition Overview Expii
4 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts

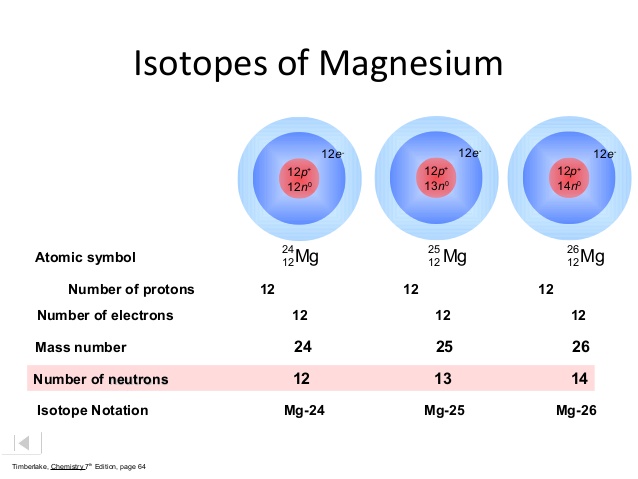



Comments
Post a Comment